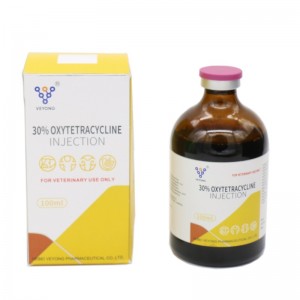
10% Oxytetracycline Injection
Video
Composition
1 ml of oxytetracycline 10% contains oxytetracycline HCI .
Indications
The use of tetracycline is indicated in systemic and local infections such as bronchopncumonia. bacterial enteritis, urinary tract infections, cholangitis, metritis, mastitis, pyodermia, Anthnix, diphtheria and CRD .
Specific indications for sheep . goat, and cattle are respiratory infections, mastitis, metritis, chlamydiosis, and infections of the cornea, conjunctiva and wound infection .
Specific indications for poultry are chronic respiratory disease ( CRD ) .
Colibacillosis and fowl cholera
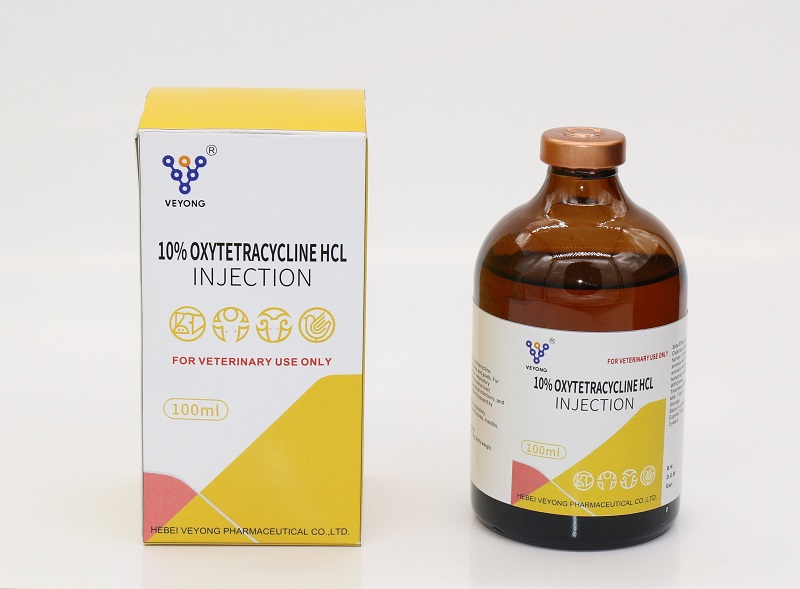
Dosage & Administration
Intramuscular injection
The general dose is : 10- 20mg / kg body weight, daily .
Adult : 1 ml / 10kg, young animals : 2 ml / 10kg body weight .
Cattle, camel, sheep, goats : a single injection at a dose of 10..mg oxytetracycline per kg of body weight or 1 ml per 10kg body weight .
Dosage Interval
Repeat every day for 3 - 5 days .
Toxicology
Acute effects due to the use of tetracycline are not often observed .
Adverse Effects
Adverse effects due to the use tetracycline are: allergic reactions, photosensitivity. and discoloration of teeth on young age group and hepatoxicity, oxytetracycline can also cause tissue titration at the site of injection .
Contraindication
A contra-indication for the use of tetracyckme is the severe liver or kidney damage and occasional hypersensitivity to tetracycline .
Therapeutic Incompatibilities
Tetracycline should not be combined with bactericidal antibiotics such as penicillines. cephalosporins. The absorption of tetracycline is inhibited when given concomitantly with
preparations containing divalent cations. Combination of tetracycline with macrolides such as tylosin, and polymyxins such as colistin, works synergistically .
Withdrawal Period
Meats : 21 days
Eggs milk :7 days
Remarks
Store below 25℃. protect from light . Keep out of reach of children .
Do not use if the solution becomes turbid or blackish .
Always consult your veterinarian before using the drug .
Hebei Veyong pharmaceutical Co., Ltd, was established in 2002, located in Shijiazhuang City, Hebei Province, China, next to the Capital Beijing. She is a large GMP-certified veterinary drug enterprise, with R&D, production and sales of veterinary APIs, preparations, premixed feeds and feed additives. As Provincial Technical Center, Veyong has established an innovated R&D system for new veterinary drug, and is the nationally known technological innovation based veterinary enterprise, there are 65 technical professionals. Veyong has two production bases: Shijiazhuang and Ordos, of which the Shijiazhuang base covers an area of 78,706 m2, with 13 API products including Ivermectin, Eprinomectin, Tiamulin Fumarate, Oxytetracycline hydrochloride ects, and 11 preparation production lines including injection, oral solution, powder, premix, bolus, pesticides and disinfectant, ects. Veyong provides APIs, more than 100 own- label preparations, and OEM & ODM service.
Veyong attaches great importance to the management of EHS(Environment, Health& Safety) system, and obtained the ISO14001 and OHSAS18001 certificates. Veyong has been listed in the strategic emerging industrial enterprises in Hebei Province and can ensure the continuous supply of products.

Veyong established the complete quality management system, obtained the ISO9001 certificate, China GMP certificate, Australia APVMA GMP certificate, Ethiopia GMP certificate, Ivermectin CEP certificate, and passed US FDA inspection. Veyong has professional team of registeration, sales and technical service, our company has gained reliance and support from numerous customers by excellent product quality, high-quality pre-sales and after-sales service, serious and scientific management. Veyong has made long term cooperation with many internationally known animal pharmaceutical enterprises with products exported to the Europe, South America, Middle East, Africa, Asia, etc. more than 60 countries and regions.


.png)
.png)
.png)
.png)