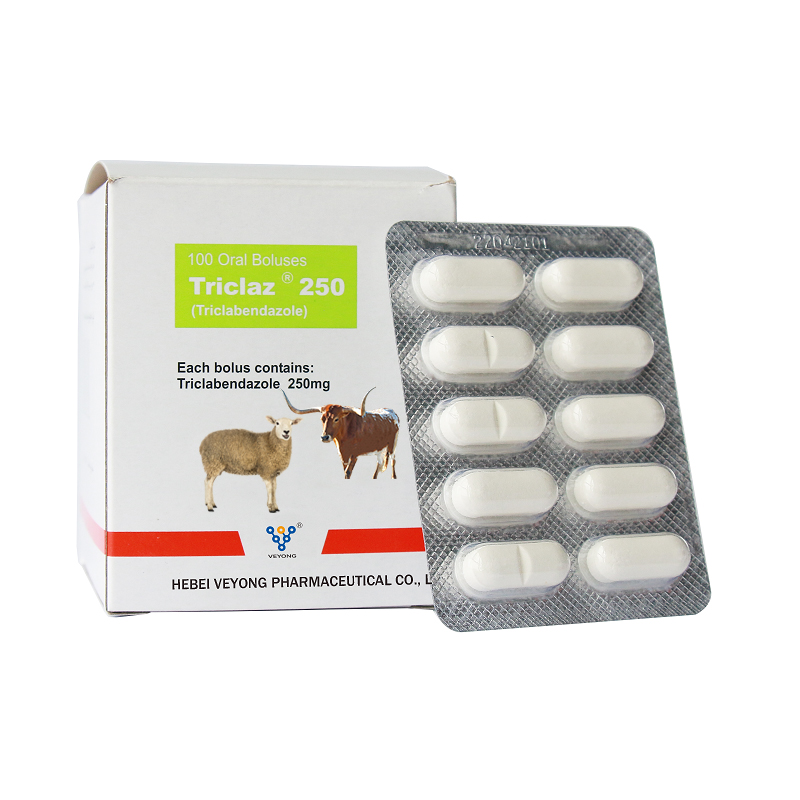
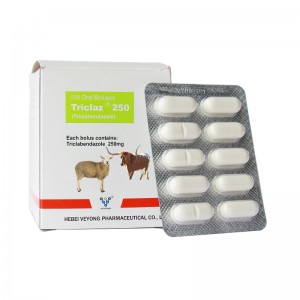
250mg Triclabendazole Bolus
Pharmacological Actions
Pharmacodynamics Triclobendazole belongs to the benzimidazole class of drugs, it is specially used to resist Fasciola hepatica, and has obvious killing effect on Fasciola hepatica of various ages. fluke medicine. After the drug is absorbed, it interferes with the microtubule structure and function of the parasite, Inhibits the release of parasite hydrolytic proteases. The effect of triclobendazole on worms varies with the concentration, such as adults at low concentrations (1 ~ 3μg/ml)
The drug still survives for 24 hours, and the activity is significantly weakened in the higher concentration (10-25μg/ml) for 24 hours; the high concentration of 25-50μg/ml completely inhibits it for 24 hours. But more sensitive to worms. At 10 μg/ml, all 24-hour activity was inhibited.
Pharmacokinetics
The bioavailability of triclobendazole is high. After oral administration of 10 mg/kg body weight in goats and sheep, the peak plasma drug reached 15 μg/ml in 24 to 36 hours, and the blood levels of triclobendazole and its metabolites were higher. The peak value of the drug is 5 to 20 times that of other benzimidazole anthelmintics, and the elimination half-life is about 22 hours. Triclobendazole is largely oxidized in sheep and rats to sulfone and sulfoxide derivatives, which bind to albumin and persist in plasma for more than 7 days. High plasma concentrations and binding to plasma albumin appear to be associated with prolonged duration of action of antifascioli. After 10 days of drug administration in sheep, about 95% of the drug is excreted in feces, 2% is excreted in urine, and less than 1% is excreted in milk.

Actions and Uses
Benzimidazole anti-fasciola drug. It is mainly used for the prevention and treatment of Fasciola hepatica infection in cattle and sheep.
Adverse reaction
No adverse reactions have been seen when used according to the prescribed usage and dosage
Precautions
(1) Disabled during milk production.
(2) It is highly toxic to fish, and the residual drug container should not pollute the water source.
(3) People who are allergic to medicines should avoid direct skin contact and inhalation when using them, wear gloves when taking medicines, and prohibit eating, drinking and smoking.
(4) Wash hands after applying
Withdrawal period
56 days for cattle and sheep
Storage
Store in the place below 30℃.
Hebei Veyong pharmaceutical Co., Ltd, was established in 2002, located in Shijiazhuang City, Hebei Province, China, next to the Capital Beijing. She is a large GMP-certified veterinary drug enterprise, with R&D, production and sales of veterinary APIs, preparations, premixed feeds and feed additives. As Provincial Technical Center, Veyong has established an innovated R&D system for new veterinary drug, and is the nationally known technological innovation based veterinary enterprise, there are 65 technical professionals. Veyong has two production bases: Shijiazhuang and Ordos, of which the Shijiazhuang base covers an area of 78,706 m2, with 13 API products including Ivermectin, Eprinomectin, Tiamulin Fumarate, Oxytetracycline hydrochloride ects, and 11 preparation production lines including injection, oral solution, powder, premix, bolus, pesticides and disinfectant, ects. Veyong provides APIs, more than 100 own- label preparations, and OEM & ODM service.
Veyong attaches great importance to the management of EHS(Environment, Health& Safety) system, and obtained the ISO14001 and OHSAS18001 certificates. Veyong has been listed in the strategic emerging industrial enterprises in Hebei Province and can ensure the continuous supply of products.

Veyong established the complete quality management system, obtained the ISO9001 certificate, China GMP certificate, Australia APVMA GMP certificate, Ethiopia GMP certificate, Ivermectin CEP certificate, and passed US FDA inspection. Veyong has professional team of registeration, sales and technical service, our company has gained reliance and support from numerous customers by excellent product quality, high-quality pre-sales and after-sales service, serious and scientific management. Veyong has made long term cooperation with many internationally known animal pharmaceutical enterprises with products exported to the Europe, South America, Middle East, Africa, Asia, etc. more than 60 countries and regions.




.png)
.png)
.png)
.png)