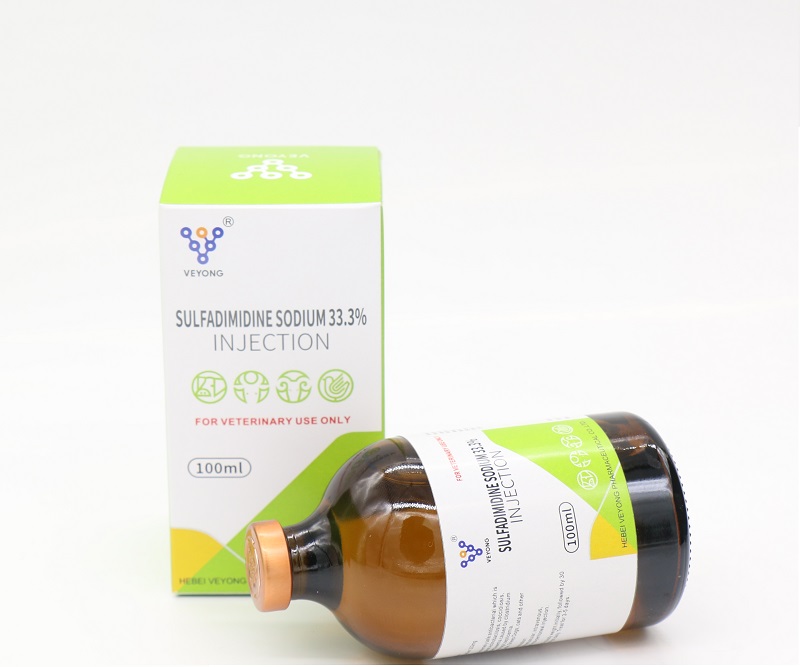
33.3% Sulfadimidine Sodium Injection
Composition
Each ml solution : Sulfadimidine sodium : 333 mg
Pharmacology and Toxicology
This product is a sulfonamide antibacterial drug, the antibacterial spectrum is similar to sulfadiazine, and it is against non-enzyme-producing Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Escherichia coli, Klebsiella, Salmonella, Shiga Enterobacteriaceae, Neisseria gonorrhoeae, Neisseria meningitidis, and Haemophilus influenzae have antibacterial effects. However, in recent years, the resistance of bacteria to this product has increased, especially Streptococcus, Neisseria and Enterobacteriaceae bacteria.
The antibacterial action mechanism of this product is similar in structure to p-aminobenzoic acid (PABA), which can compete with PABA to act on the dihydrofolate synthase in bacteria, thereby preventing PABA from being used as a raw material to synthesize folic acid required by bacteria and reducing metabolism The amount of active tetrahydrofolate, and the latter is necessary for bacteria to synthesize purine, thymidine and deoxyribonucleic acid (DNA), thus inhibiting the growth and reproduction of bacteria.

Indications
For the control of bacterial and protozoal infections in livestock and poultry. Sulfadilimine is highly effective against a wide range of bacterial , protozoal and certain rickettsial organisms. It controls coccidiosis , septicemia , pneumnia and enteritis ( salmonella , pasteurel-la , E . coli ) in cattle and calves , Necrotic enteritis in pigs , coccidio-sis , tick-bom fever in sheep and lambs , coryza , fowl cholera , coccidio - sis in poultry rabbits .
Dosage and Administration
Horses , Cattle and Camels :
15 to 30 ml per 50 kg of body weight
Sheep . goats , pigs , dogs and cats :
1.5 to 3 ml per 5 kg of body weight
Poultry and rabbits :
0.3 to 0.6 ml per kg of body weight
Cautions
- Do not use Sulfadimidine for lactating cow .
- Do not use Sulfadimidine for more than 5 consecutivedays
Mode of action and characteristics
Withdrawal period
Meet : 15 days .
Storage
Store below 30℃ in a cool dry place protected from light and heat
Hebei Veyong pharmaceutical Co., Ltd, was established in 2002, located in Shijiazhuang City, Hebei Province, China, next to the Capital Beijing. She is a large GMP-certified veterinary drug enterprise, with R&D, production and sales of veterinary APIs, preparations, premixed feeds and feed additives. As Provincial Technical Center, Veyong has established an innovated R&D system for new veterinary drug, and is the nationally known technological innovation based veterinary enterprise, there are 65 technical professionals. Veyong has two production bases: Shijiazhuang and Ordos, of which the Shijiazhuang base covers an area of 78,706 m2, with 13 API products including Ivermectin, Eprinomectin, Tiamulin Fumarate, Oxytetracycline hydrochloride ects, and 11 preparation production lines including injection, oral solution, powder, premix, bolus, pesticides and disinfectant, ects. Veyong provides APIs, more than 100 own- label preparations, and OEM & ODM service.
Veyong attaches great importance to the management of EHS(Environment, Health& Safety) system, and obtained the ISO14001 and OHSAS18001 certificates. Veyong has been listed in the strategic emerging industrial enterprises in Hebei Province and can ensure the continuous supply of products.

Veyong established the complete quality management system, obtained the ISO9001 certificate, China GMP certificate, Australia APVMA GMP certificate, Ethiopia GMP certificate, Ivermectin CEP certificate, and passed US FDA inspection. Veyong has professional team of registeration, sales and technical service, our company has gained reliance and support from numerous customers by excellent product quality, high-quality pre-sales and after-sales service, serious and scientific management. Veyong has made long term cooperation with many internationally known animal pharmaceutical enterprises with products exported to the Europe, South America, Middle East, Africa, Asia, etc. more than 60 countries and regions.





.png)
.png)
.png)
.png)