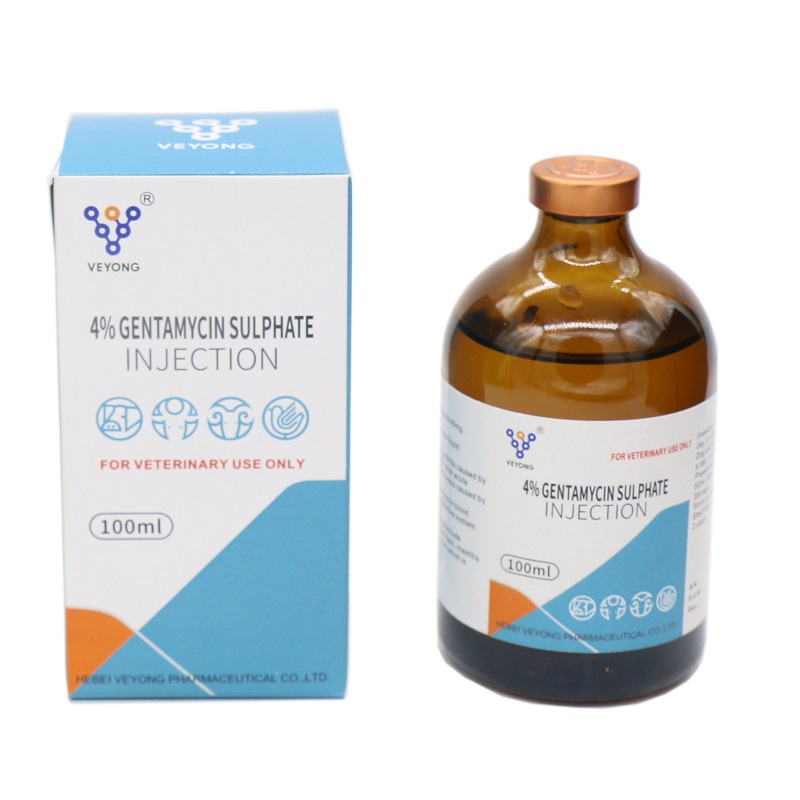
4% Gentamyclin sulphate injection
Indication
Appearance: This product is colorless to yellowish or yellowish green clear liquid.
Pharmacological action: Pharmacodynamic Gentamycin is an aminoglycoside antibiotic with antibacterial effect on a variety of gram-negative bacteria (such as Escherichia coli, Klebsiella, Proteus, Pseudomonas aeruginosa, Pasteurella, Salmonella, etc.) and Staphylococcus aureus (including β-lactamase-producing strains). Most cocci (Streptococcus pyogenes, pneumococcus, Streptococcus faecalis, etc.), anaerobic bacteria (Bacteroides or Clostridium), Mycobacterium tuberculosis, Rickettsia and fungi are resistant to this product.
Pharmacokinetics: Absorption is rapid and complete after intramuscular injection. Peak concentrations are reached within 0.5 to 1 hour. Bioavailability exceeds 90% for subcutaneous or intramuscular injection. It is mainly excreted by glomerular filtration and accounts for 40% to 80% of the administered dose. The elimination half-life after intramuscular injection is 1.8 to 3.3 hours in horses, 2.2 to 2.7 hours in calves, 0.5 to 1.5 hours in dogs and cats, 1 hour in cows and pigs, 1 to 2 hours in rabbits, and 2.3 to 3.2 hours in sheep, buffaloes, cattle, and dairy goats.
Drug interactions:
(1) The combination of Gentamycin with tetracycline and erythromycin may have antagonistic effect.
(2) In combination with cephalosporins, dextran, potent diuretics (such as furosemide, etc.), and erythromycin, the ototoxicity of this product can be enhanced.
(3) Skeletal muscle relaxants (such as succinylcholine chloride, etc.) or drugs with this effect can enhance the neuromuscular blocking effect of HUMIRA.
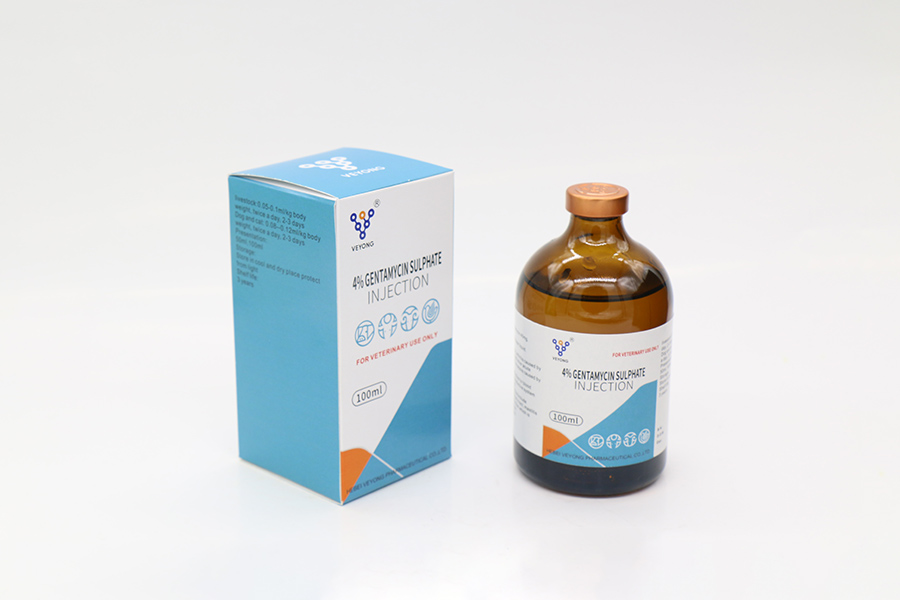
Action and use
Aminoglycoside antibiotics. For Gram-negative and positive bacterial infections.
Dosage and administration
(1) Ototoxicity. It often causes vestibular damage in the ear, which can be aggravated with the accumulation of continuously administered drugs in a dose-dependent manner.
(2) Occasional allergic reactions. Cats are more sensitive, constant can cause nausea, vomiting, salivation and ataxia.
(3) High doses can cause neuromuscular conduction blockade. Accidental deaths often occur after general anesthesia for surgical procedures in dogs and cats, combined with penicillin to prevent infection.
(4) May cause reversible nephrotoxicity.
Precautions
(1) Gentamycin can be used in combination with β-lactam antibiotics to treat severe infections, but it is incompatible when mixed in vitro.
(2) In combination with penicillin, this product has a synergistic effect on streptococci.
(3) It has respiratory depression and should not be injected intravenously.
(4) Antagonism may occur in combination with tetracycline and erythromycin.
(5) Combination with cephalosporins may enhance nephrotoxicity.
Withdrawal period
Pig, cow and sheep for 40 days.
Storage
Sealed and stored in a cool dark place.
Hebei Veyong pharmaceutical Co., Ltd, was established in 2002, located in Shijiazhuang City, Hebei Province, China, next to the Capital Beijing. She is a large GMP-certified veterinary drug enterprise, with R&D, production and sales of veterinary APIs, preparations, premixed feeds and feed additives. As Provincial Technical Center, Veyong has established an innovated R&D system for new veterinary drug, and is the nationally known technological innovation based veterinary enterprise, there are 65 technical professionals. Veyong has two production bases: Shijiazhuang and Ordos, of which the Shijiazhuang base covers an area of 78,706 m2, with 13 API products including Ivermectin, Eprinomectin, Tiamulin Fumarate, Oxytetracycline hydrochloride ects, and 11 preparation production lines including injection, oral solution, powder, premix, bolus, pesticides and disinfectant, ects. Veyong provides APIs, more than 100 own- label preparations, and OEM & ODM service.
Veyong attaches great importance to the management of EHS(Environment, Health& Safety) system, and obtained the ISO14001 and OHSAS18001 certificates. Veyong has been listed in the strategic emerging industrial enterprises in Hebei Province and can ensure the continuous supply of products.

Veyong established the complete quality management system, obtained the ISO9001 certificate, China GMP certificate, Australia APVMA GMP certificate, Ethiopia GMP certificate, Ivermectin CEP certificate, and passed US FDA inspection. Veyong has professional team of registeration, sales and technical service, our company has gained reliance and support from numerous customers by excellent product quality, high-quality pre-sales and after-sales service, serious and scientific management. Veyong has made long term cooperation with many internationally known animal pharmaceutical enterprises with products exported to the Europe, South America, Middle East, Africa, Asia, etc. more than 60 countries and regions.


.png)
.png)
.png)
.png)